Effects of Starch Level and a Mixture of Sunflower and Fish Oils on Nutrient Intake and Digestibility, Rumen Fermentation, and Ruminal Methane Emissions in Dairy Cows
by Babak Darabighane 1,Ilma Tapio 2,Laura Ventto 1,Piia Kairenius 1,Tomasz Stefański 1,Heidi Leskinen 1,Kevin J. Shingfield 1,†,Johanna Vilkki 3 andAli-Reza Bayat 1,*
Simple Summary
Methane produced by ruminants contributes to increased greenhouse gas effect. There are various nutritional strategies to reduce methane emission, such as supplementing fat or changing starch levels in the diet. Understanding the interactions of these strategies on methane emission, as well as performance, digestibility, and rumen fermentation is important. The present study aimed to assess the effects of starch level with or without a mixture of sunflower and fish oils on nutrient intake and digestibility, milk yield and composition, rumen fermentation, ruminal CH4 emissions and microbial ecology in dairy cows. Oil mixture rich in polyunsaturated fatty acids supplemented to low- or high-starch diets reduced dry matter intake and increased energy digestibility of lactating cows. High starch level improved nutrient digestibility and tended to reduce ruminal acetate:propionate ratio but did not affect rumen pH, molar propionate ratio, or ruminal CH4 emissions. Oil decreased absolute ruminal CH4 emission or tended to decrease CH4 per energy corrected milk.
Abstract
Four multiparous dairy cows were used in a 4 × 4 Latin square to examine how starch level and oil mixture impact dry matter (DM) intake and digestibility, milk yield and composition, rumen fermentation, ruminal methane (CH4) emissions, and microbial diversity. Experimental treatments comprised high (HS) or low (LS) levels of starch containing 0 or 30 g of a mixture of sunflower and fish oils (2:1 w/w) per kg diet DM (LSO and HSO, respectively). Intake of DM did not differ between cows fed LS and HS diets while oil supplementation reduced DM intake. Dietary treatments did not affect milk and energy corrected milk yields. There was a tendency to have a lower milk fat concentration due to HSO compared with other treatments. Both high starch level and oil supplementation increased digestibility of gross energy. Cows receiving HS diets had higher levels of total rumen VFA while acetate was lower than LS without any differences in rumen pH, or ruminal CH4 emissions. Although dietary oil supplementation had no impact on rumen fermentation, decreased CH4 emissions (g/day and g/kg milk) were observed with a concomitant increase in Anoplodinium-Diplodinium sp. and Epidinium sp. but a decrease in Christensenellaceae, Ruminococcus sp., Methanobrevibacter ruminantium and Mbb. gottschalkii clades.Keywords: starch; lipid; methane; microbial diversity
1. Introduction
The growing human population is boosting the demand for milk and meat as sources of animal protein, resulting in several challenges for ruminant production systems, including the need to reduce their contribution to greenhouse gas emissions. This calls for special attention to solutions for reducing methane (CH4) emissions from ruminants without negative effects on productivity. Additionally, it has been shown that 5–7% of gross energy (GE) intake is lost through CH4 production from dairy cows [1]. A number of strategies, including management, dietary approaches, and genetics have been proposed for CH4 mitigation [2,3,4]. In fact, chemical composition of the feed [5] and changing the starch content of the concentrate has been proposed as a CH4 reducing strategy [2,4]. This effect was attributed to an increase in the number of amylolytic bacteria and a drop in the number of methanogens and fibrolytic bacteria, changing ruminal volatile fatty acids (VFA) in favor of propionate production [3,4] and creating an alternative hydrogen sink to methanogenesis [6].On the other hand, unsaturated fat supplementation is another feeding strategy which not only reduces enteric CH4 production [1,2] but can improve milk monounsaturated (MUFA) and polyunsaturated fatty acid (PUFA) composition [7] with potential benefits to human health [8]. Unlike starch’s mode of action, lipid sources are not fermented in the rumen; rather, they lower fermented organic matter (OM), leading to a drop in CH4 production. Furthermore, it has been shown that medium-chain fatty acids (C14–C17) also affect the number of methanogens while unsaturated fatty acids (linoleic and α-linolenic acids) shift rumen fermentation towards production of propionate and, therefore, reduce CH4 production through toxic effects on cellulolytic bacteria and protozoa [4].Thus, our hypothesis was that higher starch level and oil supplementation would have additive effects on reducing ruminal CH4 production in dairy cows. Therefore, the present study aimed to assess the effects of starch level with or without a mixture of unsaturated fatty acids (sunflower and fish oils) on nutrient intake and digestibility, milk yield and composition, rumen fermentation, ruminal CH4 emissions, and microbial ecology in dairy cows.
2. Materials and Methods
All experimental procedures were approved by the National Ethics Committee (ESAVI/4342/04.10.03/2011, Turku, Finland) in accordance with the guidelines established by the European Community Council Directive 86/609/EEC [9].
2.1. Animals, Experimental Design and Diets
A 4 × 4 Latin square with 2 × 2 factorial arrangement of treatments was applied to four multiparous Nordic Red cows in mid-lactation (76 ± 10.4 days in milk; mean ± SD) producing 35.2 ± 2.10 kg milk/d. The cows were fitted with rumen cannula (#1C, i.d. 100 mm; Bar Diamond Inc., Parma, ID, USA) and each experimental period consisted of 14 days diet adaptation, five days as sampling period, and a 16-day washout to avoid carry-over effects to the next period. The cows were randomly allocated to the diets. Diets, formulated to be isonitrogenous, were used based on grass silage (forage to concentrate ratio 55:45 on a dry matter (DM) basis) consisting of low starch (LS) or high starch (HS) levels (16.1 and 202 g/kg DM) with 0 or 30 g of unsaturated fatty acid mixture (sunflower oil-fish oil mixture; 2:1 w/w)/kg diet DM (LSO and HSO, respectively)
The oils were stored in +4 °C until incorporated into the low-or high-starch TMR to avoid oxidation of unsaturated fatty acids and the oil replaced concentrate ingredients. The oil mixture and starch levels were selected based on our previous experiences [10] where satisfactory induction of milk fat depression was realized. One of the main objectives of this work was to study milk fat depression phenomenon (not reported in this paper). The grass silage was prepared from timothy and meadow fescue (54:46) grown at Jokioinen (60°49′ N, 23°28′ E), and ensiled with a formic acid-based ensiling additive (AIV2 plus, 5 L/t; AIV Valio Ltd., Helsinki, Finland) to allow for a restricted fermentation. In order to avoid selection of dietary components and to maintain the target forage to concentrate ratio, the diets were prepared as TMR. Experimental diets were formulated to meet or exceed metabolizable energy and protein requirements of lactating cows producing 30 kg milk/d [11] offered ad libitum to result in 5–10% refusals, and fed in four equal amounts at 06:00 h, 09:00 h, 16:30 h, and 19:30 h. Cows were kept in individual tie stalls, had free access to water and salt blocks, and were milked at 07:00 h and 16:45 h.
2.2. Feed Intake, Milk Yield and Chemical Analysis
Daily feed intake was measured by subtracting the refusals from the offered feed throughout the study but intakes during d 14–17 of each experimental period were used for statistical analysis. Representative samples of silage and concentrate ingredients during the sample collection period were used for chemical analysis. The samples were pooled within each period before chemical analysis using the standard methods described by Shingfield et al. [12]. In addition, the method proposed by Huida et al. [13] was used to correct silage DM content for the loss of volatiles. Indigestible neutral detergent fiber (iNDF) of silage and concentrates was determined by 12 d of ruminal incubation using nylon bags (60 × 120 mm, pore size 0.017 mm; Swiss Silk Bolting Cloth Mfg. Co. Ltd., Zurich, Switzerland) followed by neutral detergent fiber (NDF) analysis excluding ash. Chemical analysis of silage, concentrates, and oils plus their proportion in each diet were used to calculate chemical composition of each experimental TMR. Bomb calorimetry (1108 Oxygen bomb, Parr Instrument Co., Moline, IL, USA) was conducted to determine the GE of silage, concentrates, oils, and excreta. Milk samples were collected over 10 consecutive milking during d 15–19 of each experimental period, treated with preservative (Bronopol, Valio Ltd., Helsinki, Finland). Milk fat, crude protein (CP), and lactose were predicted using infrared analysis (MilkoScan 133B, Foss Electric, Hillerød, Denmark). Milk composition was calculated based on the weighted average of morning and afternoon milk yields.
2.3. Rumen Fermentation
On d 18 of each period and at 1.5 h intervals from 06:00 until 16:30 h, a suction pump with a Büchner flask was used to collect samples of ruminal fluid (150 mL; n = 8) through the rumen cannula. Then, pH was measured using a portable pH meter (pH 110, VWR International). Rumen liquid was filtered through two layers of cheesecloth and 5.0 mL ruminal fluid was preserved with 0.5 mL of saturated HgCl2 and 2.0 mL of 1 M NaOH to determine VFA. Furthermore, ammonia-N concentration was analyzed by collecting additional ruminal fluid (15.0 mL) preserved with 0.3 mL of 50% (vol/vol) sulfuric acid. The ruminal samples were stored at −20 °C until the time of analysis. Analysis of VFA and ammonia-N were performed as described by Shingfield et al. [12].
2.4. Apparent Total-Tract Digestibility
Feces were collected over a 72-h interval starting at 18:00 h on d 14 of each experimental period and then used to determine total tract apparent digestibility coefficients. A light harness and flexible tubing attached to the vulva was used to separate urine and feces. Representative fecal samples were collected daily and composited, dried in an oven (55 °C, 48 h). The chemical composition of fecal samples was determined using the same methods for the feed samples as described earlier.
2.5. Ruminal Gas Production
Ruminal CH4 and carbon dioxide (CO2) emissions were recorded over 6 days period (d 11–17 of each period) using sulfur hexafluoride (SF6) as a tracer marker as described by Bayat et al. [14] and validated against respiration chambers by Bayat et al. [15]. Briefly, gases in the rumen headspace were drawn continuously (1.7 mL/min) over every 24-h period into evacuated 5.5 L air-tight canisters using a capillary tubing (PEEK 1.6 mm × 0.13 mm i.d., VICI Valcro Instruments Co, Houston, TX, USA). Tubes used to collect the ruminal gas were anchored securely to the neck of the rumen cannula allowing gas collection at approximately 5 cm above the rumen mat. No correction was made for background SF6, CH4 and CO2 concentrations because cows were housed in a well-ventilated facility (72 m3/min) and fitted with custom-made sponges placed between the outer edge of the cannula flange and the abdominal wall to minimize the exchange of surrounding air with ruminal contents. Gas chromatography (Agilent 6890N, Agilent Technologies, Santa Clara, CA, USA) proposed by Regina and Alakukku [16] was applied to sub-samples of ruminal gases to analyze them in triplicates for CH4, CO2, and SF6 concentrations. Actual release rate of SF6 (1.16 ± 0.19 mg/d) in the rumen during the experiment as well as the concentrations of CH4, CO2, and SF6 measured by GC were used to calculate daily ruminal CH4 and CO2 emissions as following:CH4 (L/d) = SF6 (L/d) × [CH4]/[SF6]CO2 (L/d) = SF6 (L/d) × [CO2]/[SF6]
2.6. Microbial Analysis
Samples of ruminal digesta (2 kg) were collected from four sites (anterior dorsal, anterior ventral, posterior dorsal, and posterior ventral rumen sacs) during each period on d 15 at 15:00 h and d 17 at 09:00 h. Samples were mixed and 50 g subsample was placed into a plastic bag and snap-frozen in liquid nitrogen. Samples were stored at −80 °C until DNA extraction. Total DNA was extracted from 250 mg of ruminal digesta following Yu and Morrison [17] protocol. Rumen bacterial, archaeal and ciliate protozoan community composition was determined using 16S and 18S rRNA gene sequencing. Primers used for amplicon library preparation, sequencing conditions and sequencing data quality control were performed as described in Tapio et al. [18]. Sequencing data was further processed using Qiime v 1.9.1 [19]. Briefly, quality filtered sequences were clustered into operational taxonomic units (OTU) at 97% similarity using UCLUST [20]. Chimeric reads were filtered out using ChimeraSlayer [21]. Bacterial and archaeal OTUs taxonomy was assigned using the Greengenes 13_8 [22] and RIM-DB [23] databases, respectively. Ciliate protozoa OTUs were assigned using ciliate protozoa database [24]. Singleton OTUs were removed and the data from each sample were rarefied to the similar sequencing depth prior to further analyses.
2.7. Calculations
The difference between nutrient intake and fecal outputs was used to calculate total tract digestibility coefficients. Energy losses as CH4 were calculated using the factor 55.24 kJ/g [25]. Potentially digestible NDF (pdNDF) was calculated as the difference between NDF and iNDF. Energy corrected milk (ECM) yield was calculated according to Sjaunja et al. [26]. Methane (or CO2) emissions as proportions of organic matter intake (OMI), organic matter digestibility (OMD), and milk and ECM yields, were calculated by dividing daily CH4 emissions (g/d) by OMI, OMD, and milk and ECM yields, respectively. Methane emissions as percentage of GE intake (GEI) was calculated as CH4 energy (MJ/d) by GEI (MJ/d).
2.8. Statistical Analysis
Experimental data was analyzed by ANOVA for a 4 × 4 Latin square with a 2 × 2 factorial arrangement of treatments through the mixed procedure of SAS (version 9.2, SAS Inst. Inc., Cary, NC, USA) with a model that included fixed effects of period, starch level, oil level, and starch by oil interaction, and the random effect of cows assuming an autoregressive covariance structure fitted on the basis of Akaike information and Schwarz Bayesian model-fit criteria. The averages of data for cow within period were calculated before statistical analysis. The values reported are least square means ± SEM. The significance level p ≤ 0.05 was used to determine significant effects of starch, oil, and their interaction. In addition, probabilities at 0.05 < p < 0.10 were considered as a trend.For microbial community analysis, taxa with less than 0.01% relative abundance across all samples were filtered out before further analyses. Data was normalized by cumulative-sum scaling and log2 transformation to account for the non-normal distribution of taxonomic count data, as implemented in Calypso [27]. Microbial community alpha diversity was estimated using Shannon, Simpson diversity indices, richness and evenness estimates. Redundancy analysis (RDA) and analysis of similarity (ANOSIM), calculated based on Bray-Curtis dissimilarities, were used to identify if diet, oil or starch can be explanatory factors for the rumen microbial community composition. Permutation multivariate analysis of dispersion (Permdisp2) was used to tests whether the dispersion between the groups is significant. Analysis of variance (ANOVA) followed by subsequent pairwise comparisons with Tukey test was performed to look at diet, oil or starch effect on individual taxa. Spearman correlation was used to explore associations between rumen fermentation, methane production phenotype data and individual microbial taxa. Comparisons were counted as significant with p < 0.05. p values were further adjusted for the false discovery rate (FDR).
3. Results
3.1. Dry Matter and Nutrient Intake and Milk Yield
Intakes of DM and GE did not differ (p ≥ 0.18) between cows fed LS and HS diets while oil supplementation reduced (p < 0.01) DM and GE intakes. In comparison to LS diet, HS diet tended to increase (p = 0.087) OM intake while oil supplementation reduced (p < 0.01) OM intake. Starch intake was much greater for HS compared with LS diets as planned by design, but dietary oil inclusion reduced starch intake more in HS diet (p < 0.01 for the interaction of starch and oil). Intakes of NDF and water-soluble carbohydrates (WSC) were greater (p < 0.01) in cows fed LS than those fed HS diets. Intake of CP in cows fed HS diet was slightly greater (p < 0.05) and again oil supplementation led to a lower (p < 0.01) CP intake. As expected, intakes of saturated fatty acids (SFA), MUFA, and PUFA were greater (p < 0.01) with oil supplementation of both low- and high-starch diets. Milk and ECM yields were not influenced (p ≥ 0.11) by dietary treatments. However, ECM yield was noticeably (2.7 kg/d) yet numerically lower for HSO diet than other treatments. There was a tendency (p = 0.07 for interaction of starch level and oil supplementation) to cause lower milk fat concentration due to HSO compared with other treatments and oil supplementation reduced (p < 0.01) milk protein concentration. Inclusion of oil in the diet tended to reduce (p = 0.087) milk fat yield and reduced (p < 0.05) milk protein yield. Milk production efficiency expressed as ECM/DMI was not affected (p ≥ 0.28) by dietary treatments. Effect of dietary starch level and a mixture of unsaturated fatty acids on nutrient intake in lactating cows.
3.2. Apparent Total-Tract Digestibility
The high starch level, but not oil supplementation, increased (p < 0.01) apparent total tract digestibility of DM, OM and starch while decreased (p < 0.01) NDF and pdNDF digestibility. Both high starch level and oil supplementation increased (p ≤ 0.02) digestibility of GE and tended to increase (p = 0.058) CP digestibility. There was no interaction between starch level and oil supplementation for any of digestibility measurements. Effect of dietary starch level and a mixture of unsaturated fatty acids on nutrient digestibility in lactating cows.
3.3. Rumen Fermentation
The experimental treatments had no impact (p > 0.05) on rumen pH while HS diet tended to increase (p = 0.056) total VFA concentration compared with LS diets. However, no significant change (p = 0.21) was observed in total VFA as a result of oil supplementation. Compared with the cows receiving LS diet, cows fed HS diets had lower (p < 0.01) molar proportion of acetate and greater (p < 0.05) molar proportions of butyrate, isobutyrate, valerate, isovalerate, and caproate. The experimental treatments did not influence (p ≥ 0.18) molar proportion for propionate. Acetate to propionate ratio tended to be lower (p = 0.07) for HS compared with LS diets. Ruminal ammonia-N was greater for HS compared with LS diets (p < 0.01), and dietary oil inclusion increased ammonia more in HS diet (p < 0.05 for the interaction of starch and oil).
3.4. Ruminal CH4 and CO2 Emission
Inclusion of the oil mixture in LS and HS diets reduced (p = 0.05) daily ruminal CH4 emissions. Cows receiving oil supplements had lower CH4 emission intensity calculated as g/kg milk (p < 0.05) and g/kg ECM (p = 0.067) than their control counterparts. No difference (p ≥ 0.15) was found between the treatments in terms of CH4 emissions calculated as proportion of GE intake or g/kg OM digested. The experimental treatments were not different (p ≥ 0.16) in terms of daily ruminal CO2 emission and g/kg OM digested.
Ruminal CO2 emissions expressed as g/kg milk or ECM was greater for LS compared with other diets (p = 0.086 and 0.036 for the interaction, respectively).
3.5. Rumen Microbial Ecology
Sequencing yielded 5222–7362 good quality sequences per sample for bacteria, 1730–18,249 for archaea and 3339–12,123 for ciliate protozoa. Rumen bacterial community was represented by 18 phyla. Bacteroidetes (51–60%), Firmicutes (20–25%), Proteobacteria (1–12%), and Spirochaetes (0.9–5%) were the dominating phyla. Remaining phyla were detected at the abundance below 1%. Among bacterial genera, Prevotella was predominant in all dietary groups (40–50%) with other more abundant genera being unclassified Succinivibrionaceae (0–11%), unclassified Clostridiales (5–6%), Treponema (1–5%), unclassified Ruminococcaceae (3%), unclassified Lachnospiraceae (2–4%), Succiniclasticum (2%), Ruminococcus, Fibrobacter, CF231 and Butyrivibrio (altogether 1–2%). The remaining genera were detected at an abundance below 1%.The Archaea community was dominated by Methanobrevibacter gottschalkii (50–63%) and Methanobrevibacter ruminantium (12–24%) in all the groups. Other more dominant archaea were Methanosphaera sp. ISO3F5 (6–24%), Methanimicrococcus blatticola (1–15%) and Methanobacterium alkaliphilum (1–3%). Archaea groups belonging to the Methanomassiliicoccaceae (Mmc) family were observed at the abundance below 1%.Ciliate protozoa community was dominated by Entodinium (35–56%) in all dietary groups. Other predominant ciliate genera detected at the abundance above 5% in at least one of the diets were: Polyplastron, Ostracodinium, Metadinium, Isotricha, Eudiplodinium-Eremoplastron, Epidinium, Charonina, and Anoplodinium-Diplodinium.Dietary treatments had little effect on microbial alpha diversity estimates. Only bacterial richness was significantly (p = 0.01) reduced in high starch diets and archaeal richness tended to be numerically lower (p = 0.06) in dietary treatments with oil additive. No significant diet, oil or starch effect was observed on richness of ciliate protozoa
Beta diversity analysis was performed using RDA and ANOSIM, and identified significant clustering of bacteria with respect to diet (p = 0.006) and starch (p = 0.006). Amount of starch in the diet was also a significant explanatory factor (p = 0.05) for the clustering of ciliate protozoa. No distinct clustering of archaea was identified due to diet, oil or starch. Beta dispersion was significantly different between high and low starch bacterial communities (p = 0.009).
3.6. Taxa Affected by Diet, Oil and Starch
Diets with oil additive significantly (p < 0.05) increased abundance of Anaerovibrio sp., (Spirochaetes) PL-11B10 and ciliate protozoa Eudiplodinium-Eremoplastron (AB536716). Oil caused a significant reduction in (Bacteroidetes) RF16, (Proteobacteria) GMD14H09, Anaeroplasma sp., Prevotellaceae, (TM7) F16, Bacteroidales, and archaea belonging to Mmc. Group 8 sp. WGK1. However, only increase in Anaerovibrio sp. remained significant after correction for multiple testing using false discovery rate (FRD = 0.035) (Figure 1)
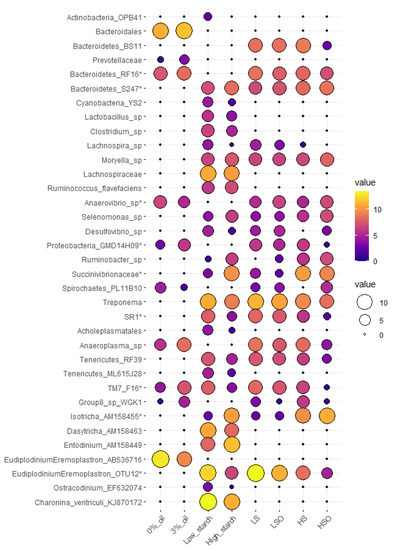
Figure 1. Mean abundance plot of microbial taxa that were significantly (p < 0.05) affected by oil, starch, or were significantly different in dietary pairwise comparisons. Microbial taxa tested are represented on Y-axis, while treatment groups tested are presented on the X-axis. Microbial values are presented as CSS normalized and log-transformed abundance data. An increase in bubble size and transition from purple to yellow color corresponds with the increase in abundance of a particular OTU. Microbial taxa with (*) are significant after FDR correction (FDR < 0.05).Diets with high starch content had significantly higher relative abundance of bacteria (Bacteroidetes) S24-7, Succinivibrionaceae spp., Ruminobacter sp., Selenomonas sp., Moryella sp., Ruminococcus flavefaciens, and ciliate protozoa Isotricha sp. and Entodinium sp. Contrary, diets with low starch content were significantly enriched in Treponema sp., (Bacteroidetes) F16, SR1, Lachnospira sp., Clostridium sp., Acholeplasmatales, Desulfovibrio sp., (Tenericutes) RF39, (Actinobacteria) OPB41, Lactobacillus sp. and ciliates affiliated with Eudiplodinium–Eremoplastron (OTU12), Charonina ventriculi, Ostracodinium sp. and Dasytricha sp. After multiple testing correction, only (Bacteroidetes) S24-7, Succinivibrionaceae sp., Isotricha sp. and Eudiplodinium–Eremoplastron (OTU12) retained significant differences (Figure 1).Diet composition had significant (p < 0.05) effect on 27 bacterial, one archaeal and seven ciliate protozoan taxa, but after FDR correction, only seven bacterial and two ciliate protozoan taxa remained significant (Figure 1). In pairwise comparisons, Anaerovibrio sp. was significantly more abundant in diets containing oil, in particular HSO diet when compared with HS, LS or LSO diets. Eudiplodinium–Eremoplastron (OTU12) was significantly more abundant in LS compared with HS or HSO diets, while Isotricha sp. was significantly more abundant in HS compared with LS or LSO diets. Bacteria (Bacteroidetes) F16, (Proteobacteria) GMD14H09, (Bacteroidetes) RF16 and SR1 were detected at the lowest abundance in HSO diet and showed significant differences between HSO and all other diets. Bacteria (Bacteroidetes) S24-7 had highest abundance in HSO diet and showed significant differences in pairwise comparisons with LS and LSO diets. Among archaea Mmc. Group 8 sp. WGK1 was more abundant (p = 0.03, FDR = 0.4) in HS diet compared with HSO or LSO diets (Figure 1)
3.7. Microbiota Association with Rumen Fermentation and Methane Production Traits
To look at the associations between rumen microbiome and rumen fermentation as well as methane production traits, we applied Spearman correlations on the microbial taxa present in all samples (n = 16 observations). From 50 bacterial, two archaeal and 25 ciliate protozoan significant associations (p < 0.05), eight bacterial and 10 ciliate protozoan correlations remained significant after correction for multiple testing using false discovery rate (FDR < 0.1).An increase in acetate was negatively correlated with (Bacteroidetes) S24-7 (FDR = 0.005), but positively with Clostridium sp. (FDR = 0.035), ciliates Dasytricha sp. (FDR = 0.053), and Charonina ventriculi (FDR = 0.057) (Figure 2). Increase in ammonia-N was positively associated with (Bacteroidetes) S24-7 (FDR = 0.011) and Moryella sp. (FDR = 0.049) but negatively associated with Treponema sp. (FDR = 0.044), (Bacteroidetes) RF16 (FDR = 0.049), Clostridium sp. (FDR = 0.049), and Charonina ventriculi (FDR = 0.07). Charonina ventriculi was negatively correlated with butyrate (FDR = 0.043) while Epidinium sp. was negatively correlated with isobutyrate (FDR = 0.062) and isovalerate (FDR = 0.016). On the contrary, Entodinium sp and Entodinium caudatum were positively correlated with isovalerate (FDR = 0.089 and 0.016, respectively). Anoplodinium–Diplodinium sp. and Epidinium sp. were negatively correlated with daily CH4 emissions (FDR = 0.064), while Christensenellaceae and Ruminococcus sp. correlation was positive. Ruminococcus flavefaciens was positively correlated with valerate (FDR = 0.063). Methanobrevibacter ruminantium and Mbb. gottschalkii clades were both positively correlated with CH4 intensity (g/kg milk) (p < 0.03, FDR = 0.12) (Figure 2).
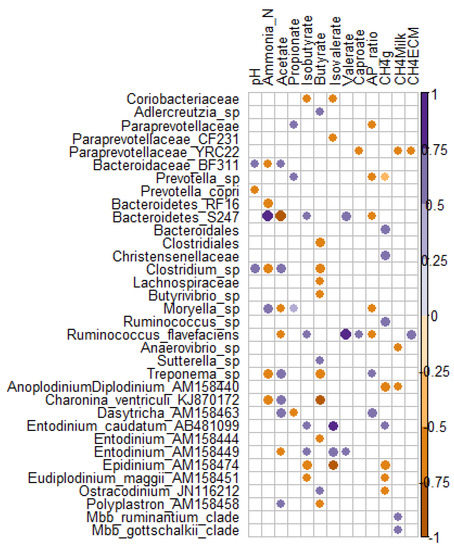
Figure 2. Spearman correlation plot between microbial taxa and rumen fermentation parameters. Only significant (p < 0.05) positive (purple) and negative (orange) correlations are presented.
4. Discussion
4.1. Dry Matter Intake, Milk Yield, and Nutrient Digestibility
The results of this experiment showed that diets with oils rich in PUFA in a moderate amount reduced DM intake, which in turn resulted in lower intakes of other nutrients (OM, CP, NDF, WSC, and starch). Similar observations have been reported previously in experiments with PUFA-rich plant oils [28,29], fish oil [30,31], or a mixture of fish oil and plant seed oils [32,33,34]. In this experiment, dietary oil supplementation reduced DM intake by 9.7% on average. In line with these results, Shingfield et al. [33] reported approximately 20.5% reduction in DM intake as a result of adding a mixture of fish and sunflower oils to corn silage-based diet (45 g oil/kg DM and 63 g ether extract (EE)/kg DM) compared with a non-supplemented diet (EE content of 33.5 g/kg DM). However, another study reported no difference in terms of DM intake of cows between the control diet based on alfalfa and corn silage (27.8 g EE/kg DM) and the same diet supplemented with mixture of fish or canola oils (20 g oil/Kg DM and 46.7 g EE/kg DM, respectively; [31]). It has been well shown that the effect of oil supplementation of a diet on DM intake can be a function of combination of including oil content and diet composition, source of oil, and type of basal diet [29,35,36].The lack of responses on milk yield due to dietary starch level and unsaturated oil mixture were expected as the diets were designed based on our previous experiences [10] to cause no effect on milk yield but lower fat and ECM yields due to the combination of high starch level and oil (i.e., HSO diet) known as milk fat depression effect. However, ECM yield was not influenced by dietary treatments despite being noticeably yet numerically lower for HSO diet (2.7 kg/d) compared with the average of other treatments. The reason for numerically lower ECM was the lower milk fat concentration (11% lower compared with LS diet).The lack of oil supplementation effect on nutrient digestibility, with exception of CP and GE digestibility is consistent with some previous findings [14,37,38].While in our experiment high-starch diet did not affect DM intake, an increase was observed for CP, OM, and starch intakes with drops in NDF and WSC intakes, which are due to differences in nutrient contents of the diets. The results of our study are consistent with the findings of Pirondini et al. [39] and Philippeau et al. [40]. In contrast, a 4.3% drop in DM intake for cows fed high- compared with low-starch diets based on grass silage (212 vs. 116 g starch/kg DM) has been reported [41]. Starch effect on feed intake can be mediated by a number of factors including starch fermentation rate, forage content of diet, amount of metabolic fuel absorbed from the rumen (for instance VFA), rumen pH, and rumen fermentation parameters [42,43]. It should be noted that the source of starch in diet and processing method can also contribute to the mixed results of different experiments. Hatew et al. [41] attributed the lower DM intake in high starch diets to increased propionate concentration in the rumen since hepatic oxidation of propionate influences DM intake [44]. Therefore, as in the aforementioned studies, no significant change was found in ruminal propionate concentration which is consistent with unaffected DM intakes.In our experiment, digestibility of DM, OM, starch, and GE was higher and CP digestibility tended to be higher in cows fed HS than those receiving LS diets. Beckman and Weiss [45] observed linear increase in DM and OM digestibility as a result of reduction in NDF:starch ratio and linked it to replacement of highly digestible carbohydrates (i.e., starch) with low-digestible carbohydrates (i.e., NDF). In the current experiment, lower NDF and pdNDF digestibility along with improved starch digestibility beside the lack of effect on rumen pH as a factor influencing fiber digestion, might reflect the competition between rumen microbial population to utilize more easily nonstructural carbohydrates when available. As shown by the results of the microbiological analysis, the abundance of Ruminobacter sp. or Selenomonas sp. increased in high starch diets while the abundance of amylolytic Prevotella or cellulolytic bacteria Ruminococcus and Fibrobacter in LS-fed cows was not significantly different from that in HS-fed cows. Another explanation might be that observed differences in fiber digestibility reflect changes in the composition of diets; in HS diet rolled barley and ground wheat were used instead of sugar beet pulp and barley feed which might have different fiber characteristics.It should be noted, however, that in some experiments where starchy feeds were replaced by fibrous by-products, there has been a confounding effect of both starch:NDF ratio and forage proportion of the diet [45]. However, in our experiment, the forage proportion in both LS and HS diets were fixed to remove such a source of difference between diets.
4.2. Methane Production and Rumen Fermentation
The oil supplementation to both low- and high-starch diets resulted in lower CH4 production compared with non-supplemented diets (475 vs. 552 g/d, on average). This is in line with the findings of the experiments that used oilseeds [29,46,47], or fish oil [30] to supplement dairy cow diets. In the current experiment, enteric CH4 emission (g/d) reduced by on average 4.7% for each 10 g/kg DM unsaturated oil in the diet. Similarly, Martin et al. [4] indicated that CH4 production reduces by 3.8% as a result of every 10 g/kg increase in dietary lipid supplementation. The most of this response in our experiment was caused by lower feed intake as CH4 yield (g/kg OMI) was not affected by the oil supplementation (average numerical reduction of 1.7% in CH4 yield). Ramin and Huhtanen [48] in a meta-analysis showed that 1 g/kg of DM increase in dietary EE concentration decreased CH4 yield by 0.043 L/kg of DM. The equivalent value in our experiment was on average 0.045 g/kg of OM intake which is very close to the reported value. Apparently both biohydrogenation of unsaturated fatty acids and amount of unfermentable organic matter introduced by oil to the diet should have caused the lower CH4 yield. Oil potential in reducing CH4 production seems to be a function of factors such as source of oil, fatty acid composition and level of supplementation in the diet [2,4]. Several mechanisms are known to influence the impact of fats and oils in reducing CH4 production including reduced OM fermentation in the rumen, unfavorable effects of C12:0 and C14:0 on protozoa community, and inhibition of methanogens by 18-carbon unsaturated fatty acids [4,49,50] and the competition for using hydrogen for biohydrogenation of unsaturated fatty acids. Patra [51] reported that C12:0 and C18:3 fatty acids are stronger inhibitors of methanogenesis compared with other fatty acids. Furthermore, as far as reduction in CH4 production is concerned, MUFA and PUFA are more effective than SFA in the diet. In this experiment, oil supplementation to both low- and high-starch diets resulted in higher intakes of both MUFA and PUFA compared with non-supplemented diets.Unaffected CH4 yield for cows fed oil-supplemented diets in our experiment is consistent with the lack of differences in rumen pH, acetate, propionate, and VFA concentration and acetate:propionate ratio, although acetate and propionate concentrations and acetate:propionate ratio decreased numerically. However, oil supplementation is expected to not only reduce CH4 production but also lower acetate:propionate ratio. The non-significant change in acetate:propionate ratio observed in our experiment can be attributed to the lower DM and concentrate intakes, and the forage portion of our experimental grass silage-based diet. Changes in rumen fermentation pattern due to oil supplementation with diets based on restrictively fermented grass silage (using the silage additive based on formic acid) may be resistant to lipid supplementation [7].Even though the intake of GE increased as a result of including oil in LS and HS diets, there were no significant differences between experimental treatments in terms of CH4 production as a percentage of GE intake while CH4 intensity calculated as g/kg milk or ECM decreased and tended to decrease by oil supplementation, respectively. In fact, the reduction in CH4 intensity indicates that net energy is partitioned more towards milk production, leading to lower CH4 intensity [3]. The values of CH4 production as a percentage of GE intake for un-supplemented diets (LS and HS; 7.25 and 7.04%) having DM intake of 23.0 and 23.5 kg/d are higher than 6.4% measured from dairy cows receiving rather similar diets with similar DM intake in respiration chambers (Bayat et al., unpublished data).Our findings showed that starch level did not influence ruminal CH4 and CO2 production (g/d) and emission intensity (g/kg milk yield). Previous studies [39,40,41] have reported lower or tendencies towards lower daily CH4 production and CH4 emission intensity with the exception of Hatew et al. [41] reporting a non-significant CH4 emission intensity due to increasing dietary starch level. Increased starch content influenced rumen fermentation parameters, with a significant decrease in molar proportion of acetate and a tendency to reduce acetate:propionate ratio. Although high starch diets are expected to reduce acetate and increase propionate molar proportions and to lower CH4 production [4], this mechanism may not invariably apply to all experimental conditions. We did not observe any differences in rumen pH between cows fed low- and high-starch diets while it has been shown that high starch content lowers rumen pH, thereby limiting growth or activity of methanogens and cellulolytic bacteria [4]. The higher rumen ammonia N concentration due to higher starch level can be attributed to slightly higher dietary CP level whereas the higher rumen ammonia N concentration with oil-containing diets might have arisen from adding urea, which is highly rumen-degradable, to the diets in an attempt to make them isonitrogenous.
4.3. Rumen Microbial Ecology
Starch level had stronger effect on bacterial richness compared with those caused by inclusion of oil in the diet. Despite reports of toxic effects of oils on microbial community and, therefore, greater expected changes in the rumen microbial composition [52], this study did not observe significant oil effect on the reduction of alpha diversity in bacterial, archaeal or ciliate protozoan communities. Effects of oil on the microorganisms may depend on type and amount of oil, type of fatty acids in diet, and type of dietary forage fed. Bayat et al. [7] showed that supplementation of dairy cow diets with plant oils like rapeseed, safflower, linseed or myristic acid reduced CH4 production, with each type of oil having a different impact on bacterial community. Furthermore, Martin et al. [37] reported that adding extruded linseed to hay-based diets or corn silage-based diets for dairy cows reduced CH4 production without a notable change in abundance of rumen methanogens and cellulolytic bacteria. Looking at individual microbial taxa, addition of oil to both low- and high starch diets in this study, provided a suitable ecological niche for lipid hydrolyzing Anaerovibrio sp. [53] and Spirochaetes order PL-11B10. PL-11B10 was detected at significantly higher abundance in the diet with myristic acid supplement [7] and outside ruminants has been found positively correlated with methane production in methanogenic oil wells [54]. Nevertheless, our understanding of PL-11B10 ecology in rumen is limited. Oil supplementation also increased relative abundance of ciliate protozoa Eudiplodinium-Eremoplastron (AB536716). Similarly, a significant increase in Eremoplastron dilobum abundance was detected in an in vitro experiment with linseed oil but not rapeseed oil additive [55], suggesting that a positive or negative oil effect is depends on protozoa species and oil type.High starch diets increased abundances of known starch utilizers like Ruminobacter sp., Selenomonas sp. or ciliate protozoa Isotricha sp. and Entodinium sp. that are involved in the utilization of non-structural polysaccharides and soluble sugars [53]. The Bacteroidetes family S24-7 was also enriched in HS diets. Current research suggests that members of S24-7 family are versatile with respect to complex carbohydrate degradation, but starch utilization trait is common to all family members and increased abundance of S24-7 is correlated with increased propionate production [56]. Inclusion of oil in LS and HS diets did not change total VFA, acetate, propionate, and butyrate but reduced CH4 production, suggesting lower H2 availability in these ruminal ecosystems. S24-7 was significantly more abundant in HSO diet, was strongly negatively correlated with acetate and tended (p = 0.054) to be positively (R = 0.49) correlated with propionate concentration in the rumen (data not shown). In addition to S24-7, also Prevotella sp., Moryella sp. and members from Paraprevotellaceae family were positively correlated with propionate concentration in the rumen (Figure 2). Given that propionate concentration was numerically the highest in HSO diet, these bacteria may have contributed to the sink in reduction of H2 availability for methanogenesis.Low starch diets were enriched with bacteria directly or indirectly involved in fiber degradation. Clostridium, Treponema, (TM7) F16, (Tenericutes) RF39, and Desulfovibrio were found to be tightly attached to switchgrass [57] or wheat straw [58] during degradation process. In co-cultures with Fibrobacter succinogenes, Treponema bryantii has been shown to utilize soluble sugars released from cellulose degradation [53]. In addition to bacteria, ciliate protozoa Ostracodinium sp. and Dasytricha sp., are known to contain cellulolytic and hemicellulolytic activities, respectively, and were significantly enriched in LS diets. Charonina ventriculi is a holotrich protozoa not frequently observed in the rumen and with limited information about its metabolism. In our experiment, Charonina ventriculi was significantly enriched in LS diets and was negatively correlated with ammonia-N and butyrate, but positively correlated with acetate concentration in the rumen. Correlation profile of Charonina ventriculi was similar to Treponema sp. and Clostridium sp. (Figure 2) suggesting that these microorganisms require similar rumen conditions for thriving or are involved in similar metabolic processes.Methanobrevibacter gottschalkii and Mbb. ruminantium were the predominant archaea without being significantly affected by starch level or oil supplement, although oil numerically reduced their abundance. Methanobrevibacter are hydrogenotrophic methanogens that convert H2 and CO2 produced by protozoa, bacteria, and fungi to CH4. In our study, numerical decreases in both Mbb. gottschalkii and Mbb. ruminantium correlated with decrease in CH4 intensity (g/kg milk) in oil supplemented diets. With higher abundances of bacteria Moryella sp., Anaerovibrio sp., (Bacteroidetes) S24-7, (Spirochaetes) PL11B10, Selenomonas sp., Ruminobacter sp., (Paraprevotellaceae) YRC22 and ciliate protozoa Anoplodinium-Diplodinium which had a tendency to be positively correlated with propionate concentration in the rumen, we can hypothesize having an ecosystem with less hydrogen available for methanogenesis.Reduced daily CH4 emissions (g/d) were positively associated with reduction in Entodinium caudatum. Although Entodinium was the most abundant ciliate protozoa in all diets and smaller Entodinium spp. have been suggested to contribute more to CH4 production compared with larger protozoa in in vitro studies [59], a deeper subdivision of Entodinium into OTUs suggests functional versatility and differences in host dependency inside this genus Contrary to our results, Belanche et al. [60] investigated holotrich protozoa role in CH4 production compared to the natural flora and concluded that holotrichs were responsible for increased methanogenesis more than the entodiniomorphids. In our study, OTU affiliated with Isotricha prostoma was negatively correlated with daily CH4 emissions (OTU detected in 10 samples and therefore not included in Figure 2), while other OTUs affiliated with holotrichs did not produce significant associations with CH4 production. Discrepancies in observations could relate to the differences in the basal diet and dietary treatment as well as host impact on the general microbial community composition.Based on the results of this experiment, it can be argued that inclusion of the mixture of fish oil and sunflower oil at 30 g/kg of diet DM does not have profound toxic effects on bacteria, archaea, or ciliate protozoa, and it is possible that the minor reduction in CH4 yield caused by inclusion of oil in diet may be linked more to the functional networks of microbiome possibly leading to a lower availability of hydrogen in the rumen which is required for CH4 production.
5. Conclusions
Overall, the results of this experiment show that starch level modified rumen fermentation and nutrient digestibility without influencing DM intake or methane emissions. Inclusion of unsaturated oil mixture (sunflower and fish oils, 2:1 w/w) reduced DM intake and some ruminal methane emission indices without influencing rumen fermentation characteristics or nutrient digestibility. The findings of this experiment show that feeding more starch originating from concentrate portion instead of fiber at a moderate level in dairy cow diets does not favor lower methane production, and oil supplementation is similarly effective on reducing methane in low- and high-starch diets. Therefore, our hypothesis that starch level and oil supplementation would have synergistic effects on CH4 emission could not be proved as increasing dietary starch level did not influence CH4 emission whereas oil supplementation did. Inclusion of moderate amount of the unsaturated oil mixture in the diet did not have profound toxic effects on bacteria, archaea, or ciliate protozoa, which is in line with the minor effect on methane yield.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ani11051310/s1, Table S1: Mean abundance of microbial taxa.
Author Contributions
Conceptualization: J.V. and K.J.S.; methodology: A.-R.B., L.V., P.K., T.S., H.L. and I.T.; validation: B.D., I.T. and A.-R.B.; formal analysis: B.D., I.T. and A.-R.B.; writing—original draft preparation: B.D., I.T. and A.-R.B.; writing—review and editing: B.D., H.L., P.K., J.V., I.T. and A.-R.B.; supervision: K.J.S., A.-R.B., I.T. and J.V.; project administration: K.J.S. and J.V.; funding acquisition: K.J.S. and J.V. All authors have read and agreed to the published version of the manuscript.
Funding
The authors acknowledge financial support from the Finnish Ministry of Agriculture and Forestry (MMM) through GreenDairy Project (no. 2908234) for the design, implementation, sample collection and data analysis. The financial aid by CEDERS project to A.-R.B. and by RumenPredict project to I.T. under the ERA-NET Co-fund scheme FACCE ERA-GAS for Monitoring and Mitigation of Greenhouse gases from Agri- and Silvi-culture is acknowledged for data interpretation and writing of the manuscript.
Institutional Review Board Statement
All experimental procedures were approved by the National Ethics Committee (ESAVI/4342/04.10.03/2011, Turku, Finland) in accordance with the guidelines established by the European Community Council Directive 86/609/EEC (European Union, 1986).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study will be made available on reasonable request from the corresponding author. Microbial sequencing data is submitted to Dryad Digital Repository with doi:10.5061/dryad.pvmcvdnk7.
Acknowledgments
The authors express their appreciation to the staff in the metabolism unit of Natural Resources Institute Finland (Luke; formerly MTT Agrifood Research Finland) for technical support, care of experimental animals, and assistance in sample collection and in the laboratory for the chemical analysis of samples. We thank Aurelie Bonin from Laboratoire d’Ecologie Alpine, CNRS, France for preparing amplicon libraries for sequencing. B.D. is deeply grateful with the CLIFF-GRADS Programme, a joint effort from CCAFS and the Global Research Alliance on Agricultural Greenhouse Gases, for the opportunity of strengthening their research skills and capabilities.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hristov, A.N.; Oh, J.; Firkins, J.; Dijkstra, J.; Kebreab, E.; Waghorn, G.; Makkar, H.; Adesogan, A.; Yang, W.; Lee, C. Special topics—Mitigation of methane and nitrous oxide emissions from animal operations: I. A review of enteric methane mitigation options. J. Anim. Sci. 2013, 91, 5045–5069. [Google Scholar] [CrossRef]
- Grainger, C.; Beauchemin, K. Can enteric methane emissions from ruminants be lowered without lowering their production? Anim. Feed Sci. Technol. 2011, 166, 308–320. [Google Scholar] [CrossRef]
- Knapp, J.R.; Laur, G.; Vadas, P.A.; Weiss, W.P.; Tricarico, J.M. Invited review: Enteric methane in dairy cattle production: Quantifying the opportunities and impact of reducing emissions. J. Dairy Sci. 2014, 97, 3231–3261. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.; Morgavi, D.; Doreau, M. Methane mitigation in ruminants: From microbe to the farm scale. Animal 2010, 4, 351–365. [Google Scholar] [CrossRef] [PubMed]
- Shibata, M.; Terada, F. Factors affecting methane production and mitigation in ruminants. Anim. Sci. J. 2010, 81, 2–10. [Google Scholar] [CrossRef]
- Hatew, B.; Cone, J.; Pellikaan, W.; Podesta, S.; Bannink, A.; Hendriks, W.; Dijkstra, J. Relationship between in vitro and in vivo methane production measured simultaneously with different dietary starch sources and starch levels in dairy cattle. Anim. Feed Sci. Technol. 2015, 202, 20–31. [Google Scholar] [CrossRef]
- Bayat, A.R.; Tapio, I.; Vilkki, J.; Shingfield, K.; Leskinen, H. Plant oil supplements reduce methane emissions and improve milk fatty acid composition in dairy cows fed grass silage-based diets without affecting milk yield. J. Dairy Sci. 2018, 101, 1136–1151. [Google Scholar] [CrossRef]
- Lanier, J.S.; Corl, B.A. Challenges in enriching milk fat with polyunsaturated fatty acids. J. Anim. Sci. Biotechnol. 2015, 6, 26. [Google Scholar] [CrossRef]
- European Union. Council Directive 86/609/EEC on the Approximation of Laws, Regulations and Administrative Provisions of the Member States regarding the Protection of Animals used for Experimental and other Scientific Purposes. Off. J. 1986, L358, 1–28. [Google Scholar]
- Kairenius, P. Role of Dietary Fish Oil and Plant Oil Supplements in Ruminal Lipid Metabolism and Fish Oil-Induced Milk Fat Depression in Lactating Cows. Ph.D. Thesis, University of Helsinki, Helsinki, Finland, 2020. [Google Scholar]
- MTT Agrifood Research Finland. Finnish Feed Tables and Feeding Recommendations. 2006. Available online: http://www.luke.fi/rehutaulukot (accessed on 13 August 2011).
- Shingfield, K.; Jaakkola, S.; Huhtanen, P. Effects of level of nitrogen fertilizer application and various nitrogenous supplements on milk production and nitrogen utilization of dairy cows given grass silage-based diets. Anim. Sci. 2001, 73, 541–554. [Google Scholar] [CrossRef]
- Huida, L.; Väätäinen, H.; Lampila, M. Comparison of dry matter contents in grass silages as determined by oven drying and gas chromatographic water analysis. Ann. Agric. Fenn. 1986, 25, 215–230. [Google Scholar]
- Bayat, A.R.; Kairenius, P.; Stefański, T.; Leskinen, H.; Comtet-Marre, S.; Forano, E.; Chaucheyras-Durand, F.; Shingfield, K. Effect of camelina oil or live yeasts (Saccharomyces cerevisiae) on ruminal methane production, rumen fermentation, and milk fatty acid composition in lactating cows fed grass silage diets. J. Dairy Sci. 2015, 98, 3166–3181. [Google Scholar] [CrossRef] [PubMed]
- Bayat, A.R.; Stefański, T.; Luukkonen, T.; Kairenius, P.; Leskinen, H.; Vilkki, J. Sulphur hexafluoride tracer technique for measuring methane directly from rumen of dairy cows validated with respiration chambers. In Proceedings of the International Symposium on Emission of Gas and Dust from Livestock, Saint-Malo, France, 21–24 May 2017; pp. 98–99. [Google Scholar]
- Regina, K.; Alakukku, L. Greenhouse gas fluxes in varying soils types under conventional and no-tillage practices. Soil Tillage Res. 2010, 109, 144–152. [Google Scholar] [CrossRef]
- Yu, Z.; Morrison, M. Improved extraction of PCR-quality community DNA from digesta and fecal samples. Biotechniques 2004, 36, 808–812. [Google Scholar] [CrossRef] [PubMed]
- Tapio, I.; Shingfield, K.J.; McKain, N.; Bonin, A.; Fischer, D.; Bayat, A.R.; Vilkki, J.; Taberlet, P.; Snelling, T.J.; Wallace, R.J. Oral samples as non-invasive proxies for assessing the composition of the rumen microbial community. PLoS ONE 2016, 11, e0151220. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; Gordon, J.I. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef]
- Haas, B.J.; Gevers, D.; Earl, A.M.; Feldgarden, M.; Ward, D.V.; Giannoukos, G.; Ciulla, D.; Tabbaa, D.; Highlander, S.K.; Sodergren, E. Chimeric 16S rRNA sequence formation and detection in Sanger and 454-pyrosequenced PCR amplicons. Genome Res. 2011, 21, 494–504. [Google Scholar] [CrossRef]
- DeSantis, T.Z.; Hugenholtz, P.; Larsen, N.; Rojas, M.; Brodie, E.L.; Keller, K.; Huber, T.; Dalevi, D.; Hu, P.; Andersen, G.L. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl. Environ. Microbiol. 2006, 72, 5069–5072. [Google Scholar] [CrossRef]
- Seedorf, H.; Kittelmann, S.; Henderson, G.; Janssen, P.H. RIM-DB: A taxonomic framework for community structure analysis of methanogenic archaea from the rumen and other intestinal environments. PeerJ 2014, 2, e494. [Google Scholar] [CrossRef]
- Kittelmann, S.; Devente, S.R.; Kirk, M.R.; Seedorf, H.; Dehority, B.A.; Janssen, P.H. Phylogeny of intestinal ciliates, including Charonina ventriculi, and comparison of microscopy and 18S rRNA gene pyrosequencing for rumen ciliate community structure analysis. Appl. Environ. Microbiol. 2015, 81, 2433–2444. [Google Scholar] [CrossRef]
- Kriss, M. Quantitative relations of the dry matter of the food consumed, the heat production, the gaseous outgo, and the insensible loss in body weight of cattle. J. Agric. Res. 1930, 40, 283–295. [Google Scholar]
- Sjaunja, L. A Nordic proposal for an energy-corrected milk (ECM) formula. In Proceedings of the 27th Session International Committee for Recording and Productivity of Milk Animals, Paris, France, 2–6 July 1990. [Google Scholar]
- Zakrzewski, M.; Proietti, C.; Ellis, J.J.; Hasan, S.; Brion, M.-J.; Berger, B.; Krause, L. Calypso: A user-friendly web-server for mining and visualizing microbiome–environment interactions. Bioinformatics 2017, 33, 782–783. [Google Scholar] [CrossRef] [PubMed]
- Bayat, A.R.; Ventto, L.; Kairenius, P.; Stefański, T.; Leskinen, H.; Tapio, I.; Negussie, E.; Vilkki, J.; Shingfield, K. Dietary forage to concentrate ratio and sunflower oil supplement alter rumen fermentation, ruminal methane emissions, and nutrient utilization in lactating cows. Transl. Anim. Sci. 2017, 1, 277–286. [Google Scholar] [CrossRef]
- Benchaar, C.; Hassanat, F.; Martineau, R.; Gervais, R. Linseed oil supplementation to dairy cows fed diets based on red clover silage or corn silage: Effects on methane production, rumen fermentation, nutrient digestibility, N balance, and milk production. J. Dairy Sci. 2015, 98, 7993–8008. [Google Scholar] [CrossRef] [PubMed]
- Fievez, V.; Dohme, F.; Danneels, M.; Raes, K.; Demeyer, D. Fish oils as potent rumen methane inhibitors and associated effects on rumen fermentation in vitro and in vivo. Anim. Feed Sci. Technol. 2003, 104, 41–58. [Google Scholar] [CrossRef]
- Vafa, T.S.; Naserian, A.A.; Moussavi, A.R.H.; Valizadeh, R.; Mesgaran, M.D. Effect of supplementation of fish and canola oil in the diet on milk fatty acid composition in early lactating holstein cows. Asian Australas. J. Anim. Sci. 2012, 25, 311. [Google Scholar] [CrossRef] [PubMed]
- Kairenius, P.; Leskinen, H.; Toivonen, V.; Muetzel, S.; Ahvenjärvi, S.; Vanhatalo, A.; Huhtanen, P.; Wallace, R.; Shingfield, K.J. Effect of dietary fish oil supplements alone or in combination with sunflower and linseed oil on ruminal lipid metabolism and bacterial populations in lactating cows. J. Dairy Sci. 2018, 101, 3021–3035. [Google Scholar] [CrossRef]
- Shingfield, K.J.; Reynolds, C.K.; Hervás, G.; Griinari, J.M.; Grandison, A.S.; Beever, D.E. Examination of the persistency of milk fatty acid composition responses to fish oil and sunflower oil in the diet of dairy cows. J. Dairy Sci. 2006, 89, 714–732. [Google Scholar] [CrossRef]
- Thanh, L.P.; Suksombat, W. Milk yield, composition, and fatty acid profile in dairy cows fed a high-concentrate diet blended with oil mixtures rich in polyunsaturated fatty acids. Asian Australas. J. Anim. Sci. 2015, 28, 796. [Google Scholar] [CrossRef]
- Rabiee, A.; Breinhild, K.; Scott, W.; Golder, H.; Block, E.; Lean, I. Effect of fat additions to diets of dairy cattle on milk production and components: A meta-analysis and meta-regression. J. Dairy Sci. 2012, 95, 3225–3247. [Google Scholar] [CrossRef] [PubMed]
- Weld, K.; Armentano, L. The effects of adding fat to diets of lactating dairy cows on total-tract neutral detergent fiber digestibility: A meta-analysis. J. Dairy Sci. 2017, 100, 1766–1779. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.; Ferlay, A.; Mosoni, P.; Rochette, Y.; Chilliard, Y.; Doreau, M. Increasing linseed supply in dairy cow diets based on hay or corn silage: Effect on enteric methane emission, rumen microbial fermentation, and digestion. J. Dairy Sci. 2016, 99, 3445–3456. [Google Scholar] [CrossRef]
- Shingfield, K.J.; Ahvenjärvi, S.; Toivonen, V.; Vanhatalo, A.; Huhtanen, P.; Griinari, J.M. Effect of incremental levels of sunflower-seed oil in the diet on ruminal lipid metabolism in lactating cows. Br. J. Nutr. 2008, 99, 971–983. [Google Scholar] [CrossRef]
- Pirondini, M.; Colombini, S.; Mele, M.; Malagutti, L.; Rapetti, L.; Galassi, G.; Crovetto, G. Effect of dietary starch concentration and fish oil supplementation on milk yield and composition, diet digestibility, and methane emissions in lactating dairy cows. J. Dairy Sci. 2015, 98, 357–372. [Google Scholar] [CrossRef]
- Philippeau, C.; Lettat, A.; Martin, C.; Silberberg, M.; Morgavi, D.; Ferlay, A.; Berger, C.; Nozière, P. Effects of bacterial direct-fed microbials on ruminal characteristics, methane emission, and milk fatty acid composition in cows fed high-or low-starch diets. J. Dairy Sci. 2017, 100, 2637–2650. [Google Scholar] [CrossRef]
- Hatew, B.; Podesta, S.; Van Laar, H.; Pellikaan, W.; Ellis, J.; Dijkstra, J.; Bannink, A. Effects of dietary starch content and rate of fermentation on methane production in lactating dairy cows. J. Dairy Sci. 2015, 98, 486–499. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.S. Effects of diet on short-term regulation of feed intake by lactating dairy cattle. J. Dairy Sci. 2000, 83, 1598–1624. [Google Scholar] [CrossRef]
- Voelker, J.; Allen, M. Pelleted beet pulp substituted for high-moisture corn: 1. Effects on feed intake, chewing behavior, and milk production of lactating dairy cows. J. Dairy Sci. 2003, 86, 3542–3552. [Google Scholar] [CrossRef]
- Allen, M.; Bradford, B.; Oba, M. Board-invited review: The hepatic oxidation theory of the control of feed intake and its application to ruminants. J. Anim. Sci. 2009, 87, 3317–3334. [Google Scholar] [CrossRef]
- Beckman, J.; Weiss, W.P. Nutrient digestibility of diets with different fiber to starch ratios when fed to lactating dairy cows. J. Dairy Sci. 2005, 88, 1015–1023. [Google Scholar] [CrossRef]
- Kliem, K.E.; Humphries, D.J.; Kirton, P.; Givens, D.I.; Reynolds, C.K. Differential effects of oilseed supplements on methane production and milk fatty acid concentrations in dairy cows. Animal 2019, 13, 309–317. [Google Scholar] [CrossRef]
- Muñoz, C.; Sánchez, R.; Peralta, A.; Espíndola, S.; Yan, T.; Morales, R.; Ungerfeld, E. Effects of feeding unprocessed oilseeds on methane emission, nitrogen utilization efficiency and milk fatty acid profile of lactating dairy cows. Anim. Feed Sci. Technol. 2019, 249, 18–30. [Google Scholar] [CrossRef]
- Ramin, M.; Huhtanen, P. Development of equations for predicting methane emissions from ruminants. J. Dairy Sci. 2013, 96, 2476–2493. [Google Scholar] [CrossRef]
- Beauchemin, K.; Kreuzer, M.; O’mara, F.; McAllister, T. Nutritional management for enteric methane abatement: A review. Aust. J. Exp. Agric. 2008, 48, 21–27. [Google Scholar] [CrossRef]
- Beauchemin, K.; McGinn, S.; Benchaar, C.; Holtshausen, L. Crushed sunflower, flax, or canola seeds in lactating dairy cow diets: Effects on methane production, rumen fermentation, and milk production. J. Dairy Sci. 2009, 92, 2118–2127. [Google Scholar] [CrossRef] [PubMed]
- Patra, A.K. The effect of dietary fats on methane emissions, and its other effects on digestibility, rumen fermentation and lactation performance in cattle: A meta-analysis. Livest. Sci. 2013, 155, 244–254. [Google Scholar] [CrossRef]
- Maia, M.R.; Chaudhary, L.C.; Figueres, L.; Wallace, R.J. Metabolism of polyunsaturated fatty acids and their toxicity to the microflora of the rumen. Antonie Van Leeuwenhoek 2007, 91, 303–314. [Google Scholar] [CrossRef]
- Hobson, P.N.; Stewart, C.S. The Rumen Microbial Ecosystem; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Shelton, J.L.; Akob, D.M.; McIntosh, J.C.; Fierer, N.; Spear, J.R.; Warwick, P.D.; McCray, J.E. Environmental drivers of differences in microbial community structure in crude oil reservoirs across a methanogenic gradient. Front. Microbiol. 2016, 7, 1535. [Google Scholar] [CrossRef] [PubMed]
- Cieslak, A.; Miltko, R.; Belzecki, G.; Szumacher-Strabel, M.; Potkanski, A.; Kwiatkowska, E.; Michalowski, T. Effect of vegetable oils on the methane concentration and population density of the rumen ciliate, Eremoplastron dilobum, grown in vitro. J. Anim. Feed Sci. 2006, 15, 15. [Google Scholar] [CrossRef]
- Ormerod, K.L.; Wood, D.L.; Lachner, N.; Gellatly, S.L.; Daly, J.N.; Parsons, J.D.; Dal’Molin, C.G.; Palfreyman, R.W.; Nielsen, L.K.; Cooper, M.A. Genomic characterization of the uncultured Bacteroidales family S24-7 inhabiting the guts of homeothermic animals. Microbiome 2016, 4, 36. [Google Scholar] [CrossRef] [PubMed]
- Piao, H.; Lachman, M.; Malfatti, S.; Sczyrba, A.; Knierim, B.; Auer, M.; Tringe, S.G.; Mackie, R.I.; Yeoman, C.J.; Hess, M. Temporal dynamics of fibrolytic and methanogenic rumen microorganisms during in situ incubation of switchgrass determined by 16S rRNA gene profiling. Front. Microbiol. 2014, 5, 307. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Wang, Y.; Li, Y.; Cheng, Y.; Zhu, W. Temporal changes of the bacterial community colonizing wheat straw in the cow rumen. Anaerobe 2018, 50, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Tapio, I.; Snelling, T.J.; Strozzi, F.; Wallace, R.J. The ruminal microbiome associated with methane emissions from ruminant livestock. J. Anim. Sci. Biotechnol. 2017, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Belanche, A.; de la Fuente, G.; Newbold, C.J. Effect of progressive inoculation of fauna-free sheep with holotrich protozoa and total-fauna on rumen fermentation, microbial diversity and methane emissions. FEMS Microbiol. Ecol. 2015, 91, fiu026. [Google Scholar] [CrossRef] [PubMed]
